
Calculating Mr from moles and mass YouTube
Mass and Mole Calculations. The mole is a unit in chemistry that tells us the amount of a substance. It gives an indication of how many particles are present in a substance.. We can also work out the masses of the products using the mass = moles x Mr equation. 0.1 x 63.5 = 6.35 g of copper. 0.05 x 44 = 2.2 g of carbon dioxide. View fullsize.

mass = Mr x moles TutorMyself Chemistry
Mass from Moles. Mr. Causey shows you step by step how to use molar mass to find the mass of a compound in moles. http://www.yourCHEMcoach.comLearn more and.

Reacting amounts calculations YouTube
The molar mass of a substance is defined as the mass of 1 mol of that substance, expressed in grams per mole, and is equal to the mass of 6.022 × 10 23 atoms, molecules, or formula units of that substance. KEY TAKEAWAY. To analyze chemical transformations, it is essential to use a standardized unit of measure called the mole.

Mole calculations 2 Mass = Mr x mole YouTube
The molar mass of a substance is the mass of one mole close mole The amount of substance that contains the same number of particles as there are atoms in 12 g of carbon-12 (contains the Avogadro's.
Early Mole Calculation Overview A Level Chemistry vlr.eng.br
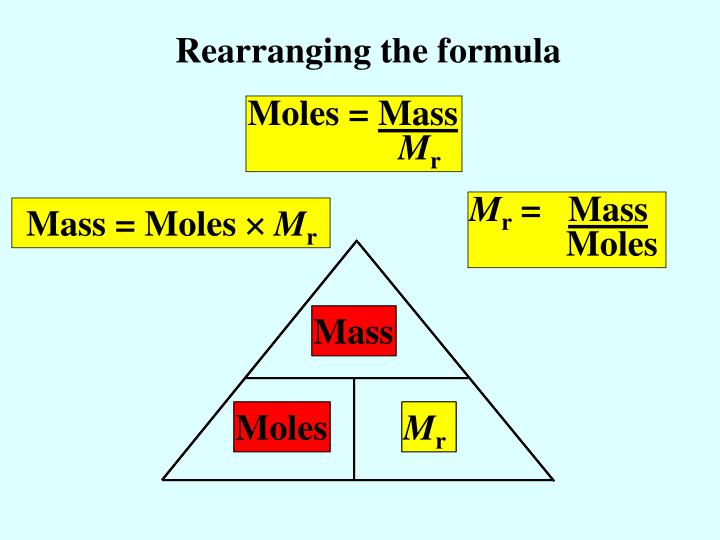
Calculating amounts in moles Key fact The amount of a given mass substance is calculated using: \ (amount=\frac {mass} {relative~atomic~or~formula~mass}\) Use Ar instead of Mr for metals.

GCSE Science (Chemistry) Calculating reacting masses from balanced equations Moles Mr Mass
A tutorial showing how to use the relative formula mass (Mr) or relative atomic mass (Ar) to calculate the mass of a substance given its moles or the number of moles in a substance.

IGCSE Edexcel Chemistry Help 1.19 carry out mole calculations using relative atomic mass (Ar
Here, Mr is the relative molar mass, also called formula weight. For normal samples from earth with typical isotope composition, the standard atomic weight or the conventional atomic weight can be used as an approximation of the relative atomic mass of the sample. Examples are: An average molar mass may be defined for mixtures of compounds. [1]
PPT Mole Calculations PowerPoint Presentation ID902714
Molar Mass. Molar mass is the mass of one mole of a substance and is expressed in grams per mole (\text{g/mol}).Chemists denote molar mass with the symbol \text{M}.It is a useful quantity in chemistry because it allows us to relate the mass of a substance to the number of particles present in it.
The mole formula triangle or pyramid isolated on a white background. Relationship between moles
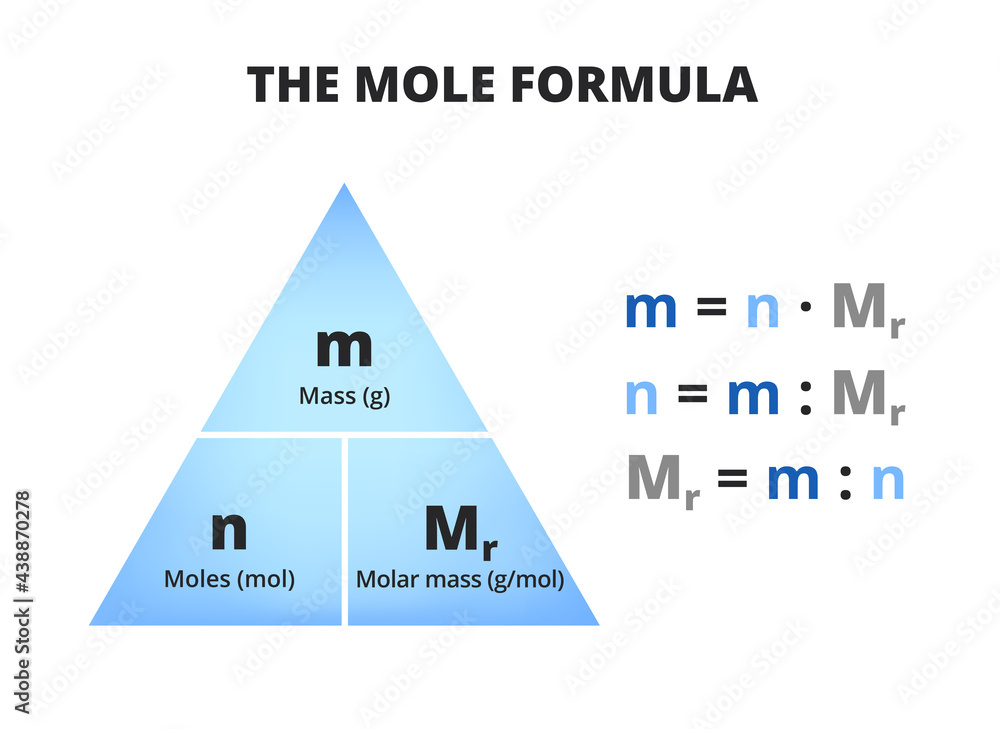
One mole is the formula mass (Mr) of any substance in grams, and it is Avogadro's number. Putting these two together, we can say that in one mole of the mass of any substance, there are 6.02 x 10 23 particles. We can use the equations to find the number of particles in any given mass of substance: Number of moles = Mass/ Mr = given number of.
IB DP Chemistry HL复习笔记1.1.5 MolesMass Problems翰林国际教育
As given to you, the molecular mass, M r, is most likely the relative molecular mass, sometimes called molecular weight, which is defined as molecular mass divided by 1 12th of the mass of a single, unbound carbon-12 atom. relative molecular mass = molecular mass 1 u For water, the relative molecular mass is M r = 18.015u 1u = 18.015
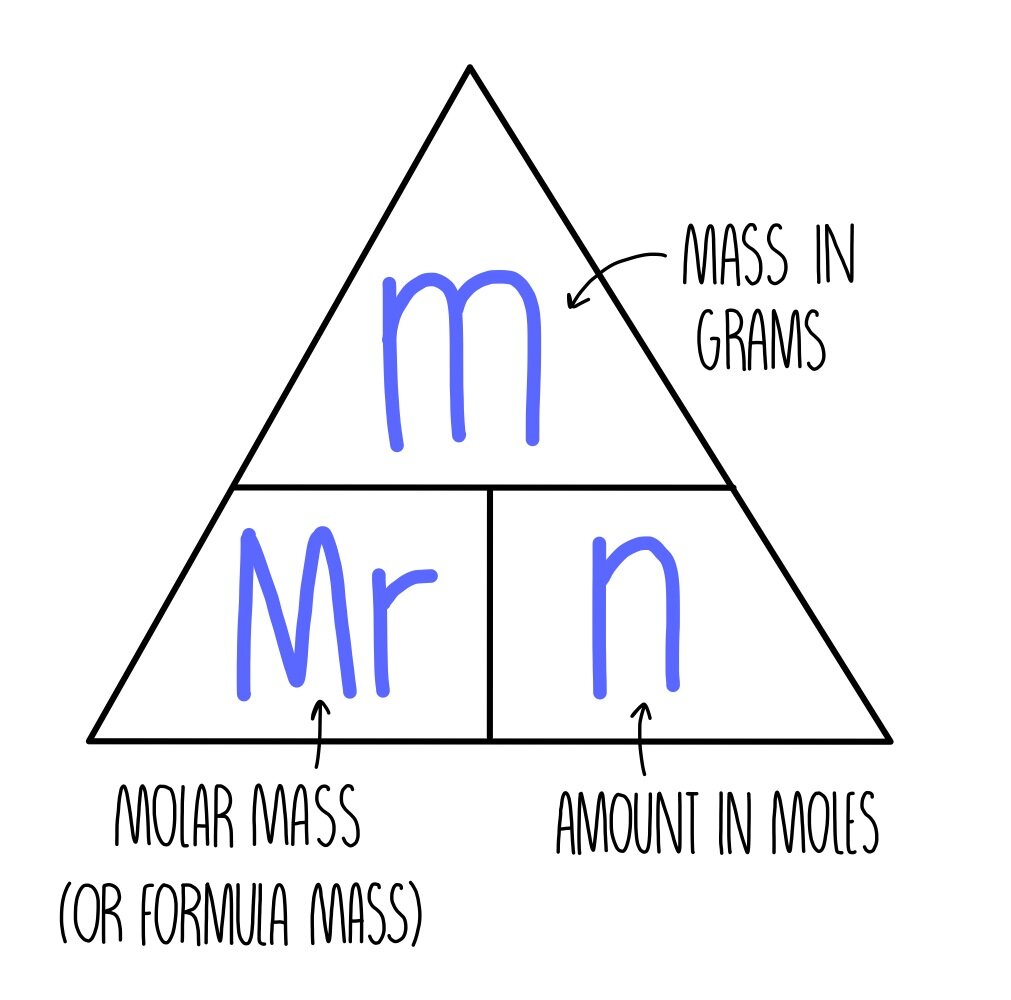
Chemical formulae, equations and calculations GCSE — the science sauce
Moles and masses , and relative formula mass are closely related. \ (moles = \frac {mass~ (g)} {M_r}\) You can imagine these properties of a substance in a triangle. You can reconfigure the.
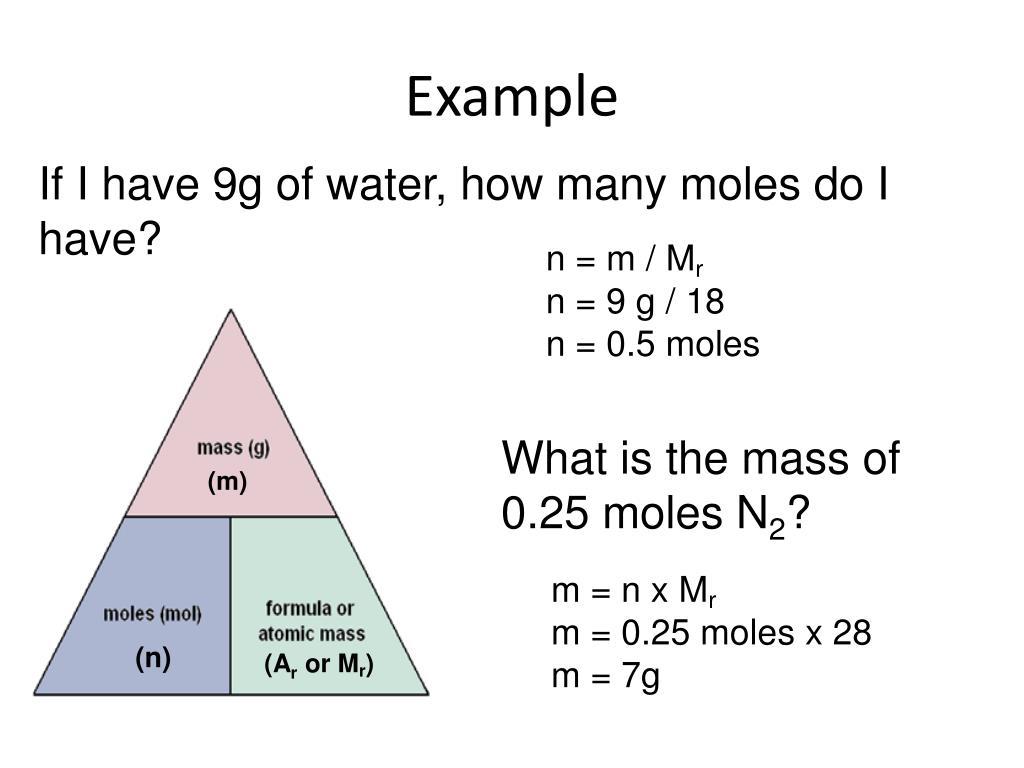
PPT Moles and reacting masses PowerPoint Presentation, free download ID2568915
The molar mass of any substance is the mass in grams of one mole of representative particles of that substance. The representative particles can be atoms, molecules, or formula units of ionic compounds. This relationship is frequently used in the laboratory. Suppose that for a certain experiment, you need 3.00 moles of calcium chloride \(\left.

How to Calculate Mass from Moles of a Compound Mr. Causey's Chemistry YouTube
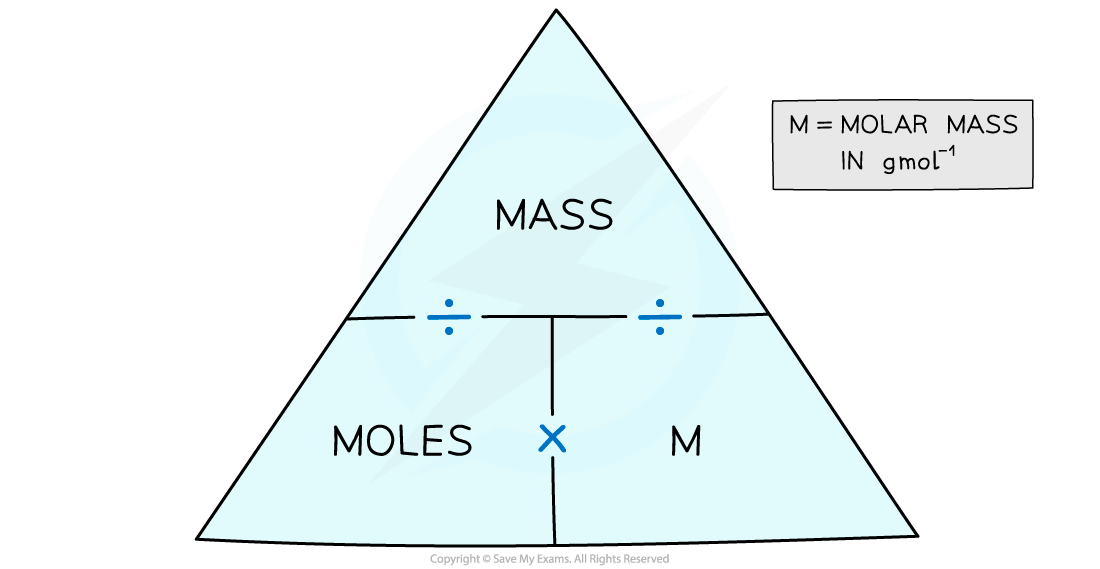
The molar mass of an element is found on the periodic table, and it is the element's atomic weight in grams/mole (g/mol). If the mass of a substance is known, the number of moles in the substance can be calculated. Converting the mass, in grams, of a substance to moles requires a conversion factor of (one mole of substance/molar mass of.
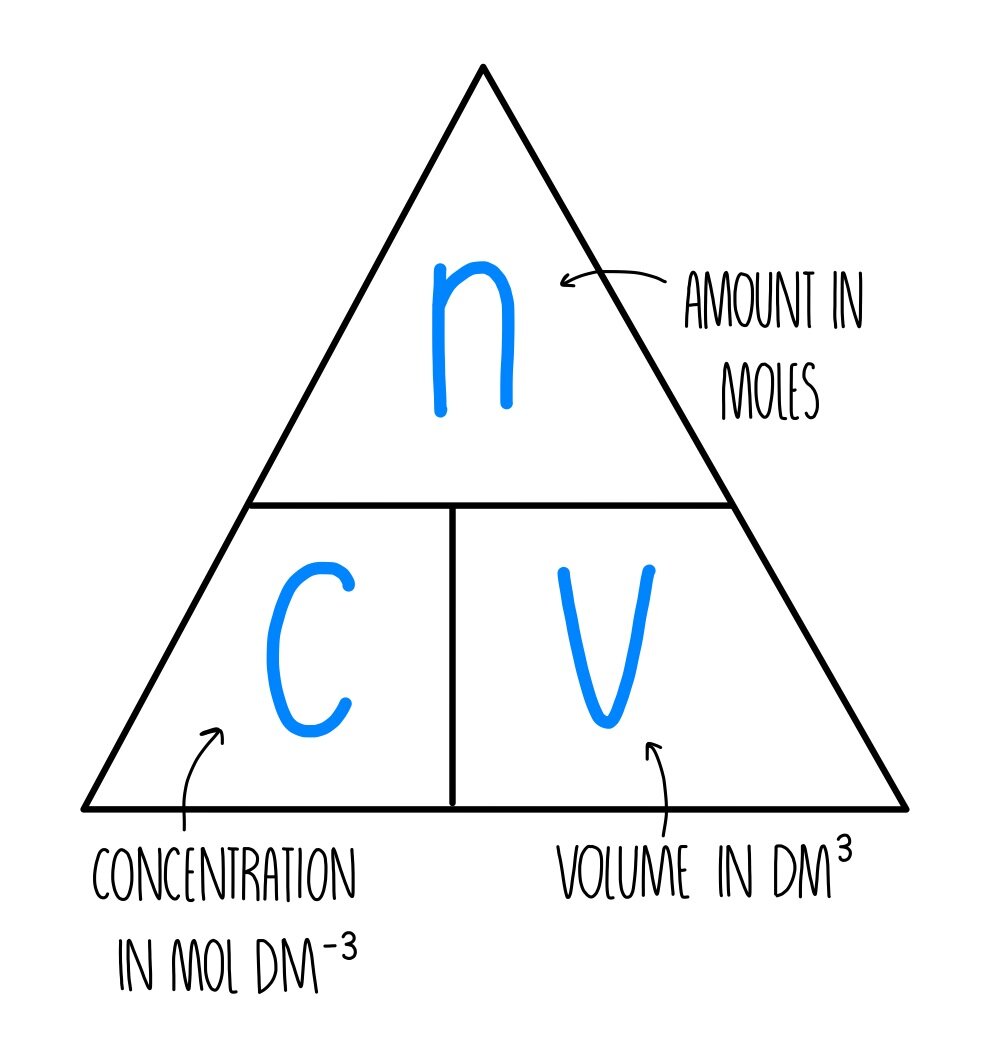
Concentration and Moles (AQA) — the science sauce
David Park. 4 years ago. First, you can calculate the molar mass of FeCl2 by adding the molar masses of Fe (55.845 g/mol) and 2 atoms of Cl (2 times (35.446 g/mol). This gives a molar mass of 126.737 g/mol. Since each mole is 126.737 grams, you multiply 3.5 mols by 126.737 grams, giving you 443.58 grams.

calculating molarity worksheet
The value of a mole is fixed, it does not change with the substance being discussed, i.e. one mole of iron, one mole of electrons, and one methane molecules both contain 6.022 \times 10^{23} particles.This number is known as the Avogadro constant and is typically give the symbols L or N A.. For any given substance, the mass of one mole (6.022 \times 10^{23} particles) of a substance will be.
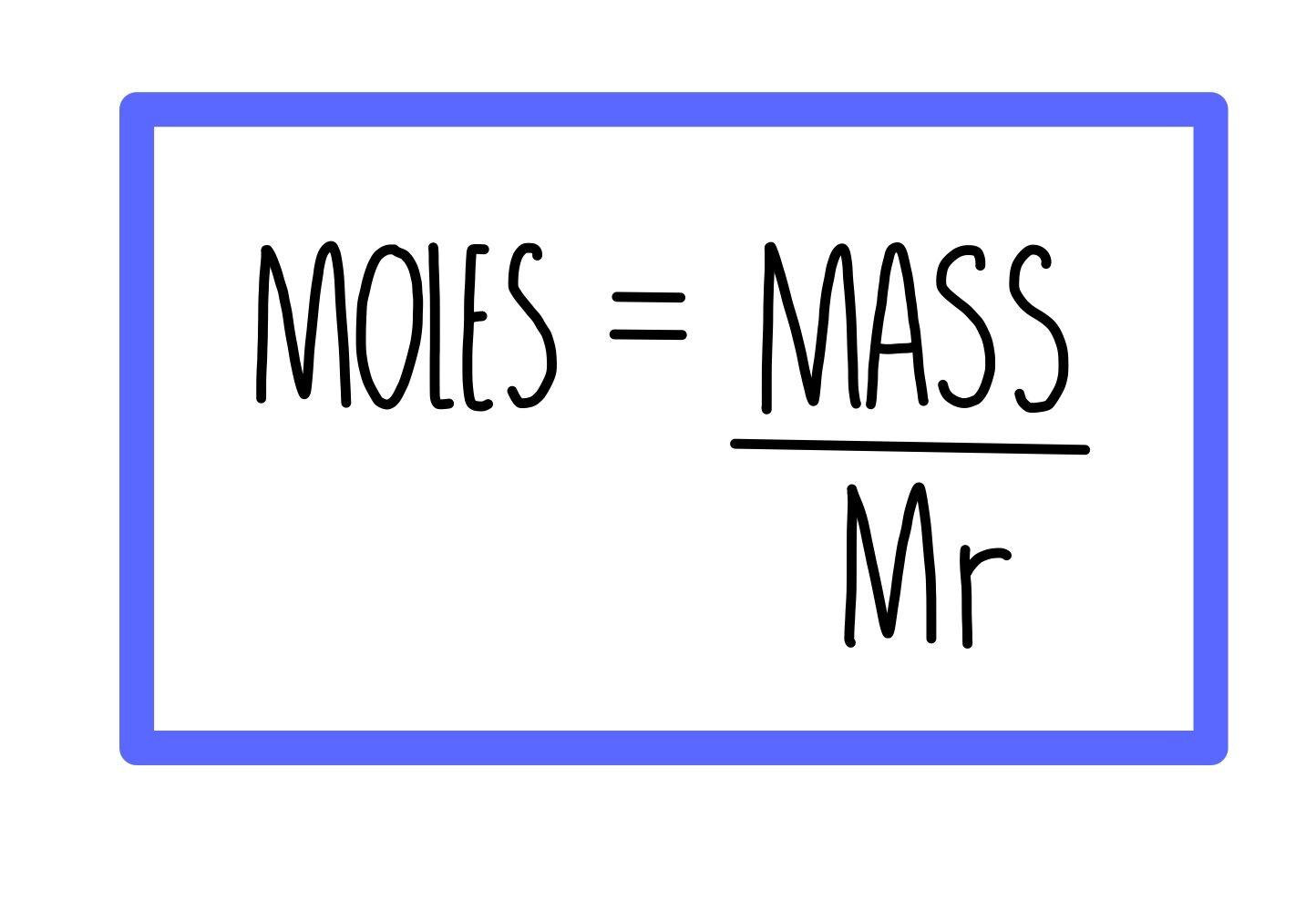
Chemical formulae, equations and calculations GCSE — the science sauce
The molar mass of a substance is defined as the mass of 1 mol of that substance, expressed in grams per mole, and is equal to the mass of 6.022 × 10 23 atoms, molecules, or formula units of that substance. Key Takeaway. To analyze chemical transformations, it is essential to use a standardized unit of measure called the mole.